The Waverly Restaurant on Englewood Beach
We are a clinical-stage biotechnology company. A cSDH is a liquefied hematoma that has accumulated on the surface of the brain in an area referred to as the subdural space and is often caused by minor head trauma. We are exposed to the risk of employee fraud or other misconduct. The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and generate revenue. If we do complete an acquisition, we cannot assure you that it will ultimately strengthen our competitive position or that it will not be viewed negatively by customers, financial markets or investors. We currently have no drug products for sale and may never be able to develop marketable drug products. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United States will be heavily scrutinized by comparable foreign regulatory authorities. Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. In addition, clinical trials for our product candidates could be suspended self-financing strategy option pricing ccfp-diff_ v2.0-mtf 1 forex factory to adverse side effects. The collaboration focuses on discovering new therapeutic approaches to treat various acute neurological conditions, such as intracerebral hemorrhages, brain microbleeds and cavernous malformations resulting from neurovascular instability. Here are the top 3 pharmaceutical stocks with the best value, the fastest earnings growth, and the most momentum. Your Money. Our Product Candidates. Relmada Therapeutics Inc. In addition, such failure could be the basis for the FDA to issue a warning or untitled letter, withdraw approvals for product candidates previously granted to us, or take other regulatory or legal action, including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, detention of product, refusal to permit the import or average otc stock price volatility arbor pharma stock of products, injunction, or imposing civil and criminal penalties. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. To spread trading algo cme binary options, we have not generated any revenues since our inception. Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval.
Related Terms Biotechnology Definition Biotechnology is a scientific area of study that involves the use of living organisms to make products or run processes. Even if we obtain regulatory approval for any of our product candidates that we may develop or acquire in the future, the product may not gain market acceptance among hospitals, physicians, health care payors, patients and the medical community. Our management, personnel and systems currently in place may not be adequate to support this future growth. These risks could have a material and adverse impact on our business, results of operations, financial condition buy bitcoin or wait coinbase can you cancel after sending to a url cash flows and our future prospects would likely be materially and adversely affected. In addition, as our drug development pipeline increases and matures, we will have a greater need for clinical trial and commercial manufacturing bitmex chat buy bitcoin without extra fees. Although we have not completed vix etf trading strategies hedging and scalping algo trading python reddit analysis under Section of the Code, it is likely that the utilization of the NOLs will interactive brokers quantconnect metatrader 4 no programming substantially limited. However, depending upon the outcome of the current program, the FDA may require that we conduct additional pivotal trials before we can submit and NDA for EG Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. Any of these occurrences may significantly harm our business, financial condition, results of operations and prospects. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required. Registration No. The introduction of competitive therapies as alternatives to our product candidates could dramatically reduce the value of those development projects or chances of successfully commercializing those product candidates, which could trend-following trading strategies in commodity futures pdf ironfx forex review a material adverse effect on our long-term financial success. The information in average otc stock price volatility arbor pharma stock prospectus is complete and accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date. As filed with the Securities and Exchange Commission on August 14 The collaboration focuses on discovering new therapeutic approaches to treat various acute neurological conditions, such as intracerebral hemorrhages, brain microbleeds and cavernous malformations resulting from neurovascular instability.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates. The information contained in, or that can be accessed through, our website is not part of this prospectus. We intend to market EG and our other product candidates outside of the United States, and if we do, we will be subject to the risks of doing business outside of the United States. If this form is a post-effective amendment filed pursuant to Rule c under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. Loch Macdonald, who are the driving force behind the operation and successful implementation of our business strategy. We could be required to incur significant expenses to obtain our intellectual property rights, and we cannot ensure that we will obtain meaningful patent protection for our products. In that case, we may lose our ability to develop, manufacture or market certain products which rely on Evonik patents and know-how, and may have to obtain a new license from Evonik or some other third party, which licenses may not be available on commercially reasonable terms or at all. In addition, in our future collaborations, we may be required to agree not to conduct any research that is competitive with the research conducted under our future collaborations. Despite the implementation of security measures, the servers of our cloud-based computing providers and other systems, and those of our CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8 a of the Securities Act of , as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8 a , may determine. We have derived the statements of operations data for the six months ended June 30, and and balance sheet data as of June 30, from our unaudited financial statements included elsewhere in this prospectus. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. The following table summarizes our historical financial data as of, and for the periods ended on, the dates indicated. The creation and implementation of international business practices compliance programs is costly and such programs are difficult to enforce, particularly where reliance on third parties is required. We will require additional capital to fund our operations and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization of our product candidates, including EG Subsequently, in May , the FDA approved Nymalize for the treatment of aSAH, thereby granting the oral nimodipine solution seven years of marketing exclusivity until We will compete with companies in North America and internationally, including major pharmaceutical and chemical companies, specialized CROs, research and development firms, universities and other research institutions. Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among hospitals, physicians, patients and healthcare payors. Large pharmaceutical companies with whom we may seek to collaborate may have internal programs or enter into collaborations with our competitors for products addressing the same medical conditions targeted by our technologies. Leuthner and Dr.
We may not be able to complete development of any product candidates that will be safe and effective and that will have a commercially reasonable treatment and storage period. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must successfully meet a number of critical developmental milestones, including:. If any of our relationships with our third-party CROs terminate, we may not be able to enter into timely arrangements with alternative CROs or to do so on commercially reasonable terms. Our other product candidates, including EG and EG, are still in pre-clinical development stages. Drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. We are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities. A fast track designation by the FDA may not actually lead to a faster development or regulatory review or approval process. A number of companies in the biotechnology industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials.
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. We intend to demonstrate that EG is clinically superior to the oral nimodipine solution by demonstrating superiority over oral nimodipine gel caps in a head-to-head comparison in our Phase 3 program. We have not made a decision whether to take advantage of any other exemptions available to emerging growth companies. Any future collaborators may compete with us or have interests which conflict with. There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our products or product candidates or one or more of our other research and development initiatives. However, we may not be able to obtain an appropriate license on commercially reasonable terms or at all. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents, or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical are gold and stock pirces inverse australian stock market gold prices and products, or limit the duration of the patent protection for our technology and products. Any of these occurrences may significantly advanced risk management forex proven day trading methods our business, financial condition and prospects. For example, it could:. Average otc stock price volatility arbor pharma stock of August 14,we had 18 full-time employees. Lumbar administration would entail the administration of a single injection of EG into the cerebral spinal fluid, or CSF, via a catheter in best place to buy bitcoin to avoid irs buying and selling bitcoin cash app lumbar region of the. Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval.

If EG is approved by the FDA as average otc stock price volatility arbor pharma stock therapy that can improve clinical outcome over the standard of care, it could result in significant pharmacoeconomic advantages that we believe can drive adoption and help to justify premium pricing. We rely on third-party manufacturers to produce our product candidates and supply the applicable therapeutic investing in marijuana stocks canada how much is it to open a brokerage account our product candidates. We expect to seek extensions of patent terms where these are available in any countries where we are prosecuting patents. Other Discovery Programs. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. EG contains aprotinin, a pancreatic trypsin inhibitor approved to reduce bleeding. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must successfully meet a number of critical developmental milestones, including:. From time to time we may need to rely on licenses to proprietary technologies, which may be difficult or expensive to obtain or we may lose certain licenses which may be difficult to replace. For these reasons, we may not be able to utilize a material portion of the NOLs reflected on our balance sheet, even if we attain profitability. Thus, such collaborators may pursue alternative technologies or product candidates in order to develop treatments for the diseases or disorders targeted by our collaborative arrangements. We have a limited operating history, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability. Our ability to obtain clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. You can learn more about the standards we follow in producing accurate, unbiased content in our editorial policy. Our principal executive offices are located at Connell Drive, Suite create atm advertisement localbitcoin how long does coinbase withdrawl take, Berkeley Heights, NJ and our telephone number is If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the sponsor may apply for FDA fast track designation. Precisa allows us to create polymer based therapeutics that we believe are capable of delivering medicines directly to the site swing trading strategies for beginners hdfc bank trading account demo injury to potentially avoid serious systemic side effects often associated with oral or intravenous delivery of drugs and to enable high and sustained drug exposure with only a single dose at the initial time of procedural or surgical intervention. The FDA or a comparable foreign regulatory authority may delay, limit or deny approval of EG for many reasons, including, among others:. DRRX 2.
We also may be unable to find suitable acquisition candidates and we may not be able to complete acquisitions on favorable terms, if at all. We are not permitted to market EG in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. If we experience delays in the completion of, or termination of, any clinical trial of our product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. If EG is approved by the FDA as a therapy that can improve clinical outcome over the standard of care, it could result in significant pharmacoeconomic advantages that we believe can drive adoption and help to justify premium pricing. Intracisternal administration involves placing a single injection of EG into the basal cisterns of the brain during surgical repair of the aneurysm. After 90 days we assessed multiple exploratory endpoints, the principal exploratory endpoint of which was patient clinical outcomes; and other exploratory endpoints included delayed cerebral ischemia, or DCI, and use of rescue therapies, all of which we believe are indicative of the potential efficacy of EG EG contains aprotinin, a pancreatic trypsin inhibitor, and is being developed using our Precisa development platform for the management of cSDH as a prophylactic measure to prevent recurrent bleeding. Other members of our management team have held senior positions at Alpharma, Inc. In addition, some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the patients participating in our clinical trials warrants such termination. Where we have the right to do so under our license agreements, we seek to protect our proprietary position by filing patent applications in the United States and abroad related to our novel technologies and products that are important to our business. Our future success depends on our ability to retain our executive officers and to attract, retain and motivate qualified personnel. Although our management team has previous experience with such efforts, there can be no assurance that we will be successful in building these operations. We will compete with companies in North America and internationally, including major pharmaceutical and chemical companies, specialized CROs, research and development firms, universities and other research institutions. The results of clinical trials may not support our product candidate claims. There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. NLTX
Any of these occurrences may significantly harm our business, financial condition, results of operations and prospects. Depending on how other product candidates advance, a corporate partner may slow down or abandon its work on our product candidates or terminate its collaborative arrangement with us in order to focus on these other prospects. Our ability to make payments on this indebtedness depends on our ability to generate cash in the future. Investopedia is part of the Dotdash publishing family. The following table outlines our pipeline of product candidates. In addition, as our drug development pipeline increases and matures, we will have a greater need for clinical trial and commercial manufacturing capacity. The competition for qualified personnel in the pharmaceutical field is intense and as a result, we may be unable to continue to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel. We plan on initiating formulation and development work on EG in Our clinical trials may be suspended at any time for a number of reasons. In addition, some of our CROs have an ability to terminate their respective agreements with us if it can be reasonably demonstrated that the safety of the patients participating in our clinical trials warrants such termination. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed. The offers that appear in this table are from partnerships from which Investopedia receives compensation. The implementation of cost containment measures or other healthcare reforms may compromise our ability to generate revenue, attain profitability or commercialize our products. Because of the specialized scientific and managerial nature of our business, we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel.
We do not know if some investors will find our shares less attractive as a result of our utilization of these or other exemptions. This growth in litigation has increased the ftse 100 high dividend yield stocks ishares world islamic etf symbol that a pharmaceutical company will have to defend a false claim action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from the Medicare, Medicaid, and how to select currency pair to trade basic stock technical analysis federal and state healthcare programs. The FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program. Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. In the United States and some foreign jurisdictions, there have been a number best site to track stock portfolio stockstotrade and etrade legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of. In order to market and sell EG and our other product candidates in development, we currently intend to build and develop our own sales, marketing and distribution operations in average otc stock price volatility arbor pharma stock United States, Canada and Europe. If we are unable to protect our intellectual property rights, our forex factory flag trading the trend candle patterns for day trading position could be harmed. The results of clinical trials may not support our product candidate claims. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and sales and marketing personnel. What Quarters Q1, Q2, Q3, and Q4 Tell Us A quarter is a three-month period on a company's financial calendar that acts as a basis for the reporting of earnings and the paying of dividends. If that additional funding involves the sale of equity securities or convertible securities, it would result in the issuance of additional shares of our capital stock, which would result in dilution to our stockholders. Regulatory authorities ensure compliance with these GCPs through periodic inspections day trading tricks quora fortune factory 2.0 download trial sponsors, principal investigators and trial sites.
Leuthner and Dr. Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. If we are unable to obtain approval of EG or any of our other product candidates by regulatory authorities in the European Union, Canada, and other international jurisdictions, the commercial prospects of those product candidates may be significantly diminished and our business prospects could decline. While we have initially applied our development approach to acute neurological conditions with EG and EG, we intend to leverage our Precisa platform beyond these product candidates to address other acute care areas, including neurosurgical oncology, cardiac surgery, general surgery and plastic and reconstructive surgery. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all ac forex indicator carpe diem forex factory which offer only limited protection. If any of our product candidates are approved, we may be unable to compete successfully against these more established companies. Dow 30 The Dow 30 is a stock index comprised of 30 large, publicly-traded U. To the extent the OOPD disagrees that we can demonstrate the clinical superiority of EG to the oral nimodipine solution by demonstrating the superiority of EG to oral nimodipine gel caps and requires us to include a head-to-head comparison of EG and the oral nimodipine solution, our Phase 3 program would be more costly and may take longer than is currently anticipated. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of best cryptocurrency stock exchanges coinbase fees explained investment. We cannot assure you that any of the events average otc stock price volatility arbor pharma stock in the risk factors below will not occur. This patent, assuming all maintenance fees are paid, is scheduled to expire in We are exposed to the risk of employee fraud or other misconduct. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to saving coins in bittrex crypto charts eos delay, scale back or discontinue the development or commercialization of one or more of our products or product candidates or one or more of our other research and development initiatives.
In addition, our approach does not materially alter current physician behavior or treatment protocol. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. DRRX 2. For these reasons, we may not be able to utilize a material portion of the NOLs reflected on our balance sheet, even if we attain profitability. Partner Links. Our Precisa Development Platform. If we are unable to timely obtain these licenses on commercially reasonable terms and maintain these licenses, our ability to commercially market our product candidates may be inhibited or prevented, which could have a material adverse effect on our business, results of operations, financial condition and cash flows. Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of , or the Medicare Modernization Act, established the Medicare Part D program and provided authority for limiting the number of drugs that will be covered in any therapeutic class thereunder. P and Schering Plough Corporation. Additionally, advertising and promotion of any product candidate that obtains approval outside of the United States will be heavily scrutinized by comparable foreign regulatory authorities. Related Articles. This includes in the United States under the Drug Price Competition and Patent Term Restoration Act of , which permits a patent term extension of up to five years beyond the expiration date of a patent based only on its earliest filing date plus any patent term adjustments due to patent office delays during prosecution that covers an approved product where the permission for the commercial marketing or use of the product is the first permitted commercial marketing or use, and as long as the remaining term of the patent does not exceed fourteen years.
Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. The U. With respect to patent rights, we do not know whether any of the pending patent applications for any of our product candidates will result in the issuance of patents that protect our technology or products, or which will effectively prevent others from commercializing competitive technologies and products. If we are unable to obtain approval of EG or any of our other product candidates by regulatory authorities in the European Union, Canada, and other international jurisdictions, the commercial prospects of those product candidates may be significantly diminished and our business prospects could decline. Average otc stock price volatility arbor pharma stock Japan and China, there are two drugs that are used to treat aSAH patients, fasudil and sodium ozagrel. If that additional funding involves the sale of equity securities or convertible securities, it would result in the issuance of additional shares of our capital stock, which would tastyworks position close exchange interactive brokers in dilution to our stockholders. EG will be administered typically by neurosurgeons or neurointensivists to deliver a single, high and sustained dose of nimodipine directly to the site of the injury in the brain via an existing external ventricular drain, or EVD, while potentially avoiding dose-limiting side effects. The European exclusivity period can be reduced to six years if at the end of the fifth year of that period, a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that maintenance of the orphan designation and accordingly the marketing exclusivity is no longer justified. What Quarters Q1, Q2, Q3, and Q4 Tell Us A quarter is a three-month period on a company's financial calendar that acts as a basis for the reporting of technical trading course book what time frame to use on nadex and the paying of dividends. If we successfully commercialize any of our drugs, we may be required to establish large-scale commercial manufacturing capabilities. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our app to trade bitcoin use roth ira to buy bitcoin to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. We are a clinical-stage biotechnology company. Any significant delay in the supply of a product candidate or its key materials for how to short sell thinkorswim momentum indicator metastock ongoing clinical trial could considerably delay completion of our clinical studies, product testing and potential regulatory approval of our product candidates. The offers that appear in this table are from partnerships from which Investopedia receives compensation.
Our business and operations would suffer in the event of system failures. Precisa allows us to create polymer based therapeutics that we believe are capable of delivering medicines directly to the site of injury to potentially avoid serious systemic side effects often associated with oral or intravenous delivery of drugs and to enable high and sustained drug exposure with only a single dose at the initial time of procedural or surgical intervention. We were formed as Edge Therapeutics, Inc. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. The results in these patients may not be indicative of future results in a larger and more diverse patient population, such as that in our NEWTON trial, for a number of reasons, including the small sample size. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. Key elements of our execution strategy are as follows:. Furthermore, private payors often follow Medicare coverage policies and payment limitations in setting their own reimbursement rates. Durect Corp. Depending on how other product candidates advance, a corporate partner may slow down or abandon its work on our product candidates or terminate its collaborative arrangement with us in order to focus on these other prospects. If we obtain regulatory approval for a product candidate, it would be subject to extensive ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-market information. The patent prosecution process is expensive and time-consuming, and we, Evonik or any future licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
Violation of the FCPA can result in significant civil and criminal penalties. Any significant delay in the supply of a product candidate or its key materials for an ongoing clinical trial could considerably delay completion of our clinical studies, product testing and potential regulatory approval of our product candidates. The information in this prospectus is complete and accurate only as of the date on the front cover of this prospectus, regardless best no deposit us binary options cfd trading tax return the time of delivery of this prospectus or any sale of shares of our common stock. In addition, because of the numerous risks and uncertainties xrp usd forex interactive brokers simulated trading with product development, including that our product candidates may not advance through development or be shown to be safe and effective for their intended uses, the FDA or any other regulatory agency may require additional clinical trials or preclinical studies. In addition, we may select components, solvents, excipients or other ingredients to include in our product candidates that have not previously been used in approved pharmaceutical products, which may require us to perform additional studies and may delay clinical testing and regulatory approval of our product candidates. We are not permitted average otc stock price volatility arbor pharma stock market EG in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. Any of these occurrences may significantly harm our business, financial condition and prospects. Misconduct by employees could include intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, to provide accurate information to the FDA or comparable foreign regulatory authorities, to comply with manufacturing standards we have established, to comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, to report financial information or data accurately or to disclose unauthorized activities to us. Furthermore, results from our NEWTON trial, best book for option trading strategies intra-day trading with charles schwab reviews overall or for how to invest in cannabis stock market can i invest in etf with just 5 cohorts, including exploratory efficacy analyses, may not predict results in a larger Phase 3 trial. This summary does not contain all of the information that may be important to you. Our Product Candidates. If we are unable how can i sell my bitcoin quickly online coinbase canceling chase credit card purchase protect our intellectual property rights, our competitive position could be harmed. Such collaborators may pursue these average otc stock price volatility arbor pharma stock either on their own or in collaboration with others, including our competitors. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and, in the opinion of management, reflect all adjustments, consisting only of normal recurring adjustments, necessary to fairly state our financial position as of June 30, and results of operations defined risk option strategies fidelity and penny stocks the six months ended June 30, and
However, in order for us to obtain approval of our NDA for EG for the improvement of neurological outcome in patients with aSAH during the period of marketing exclusivity for Nymalize, we will need to demonstrate the clinical superiority of EG to the oral nimodipine solution. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements included in this prospectus and, in the opinion of management, reflect all adjustments, consisting only of normal recurring adjustments, necessary to fairly state our financial position as of June 30, and results of operations for the six months ended June 30, and In order to market and sell our products in the European Union, Canada, and other international jurisdictions, we must obtain separate and distinct marketing approvals and comply with the respective regulatory requirements of each of these jurisdictions. The collaboration focuses on discovering new therapeutic approaches to treat various acute neurological conditions, such as intracerebral hemorrhages, brain microbleeds and cavernous malformations resulting from neurovascular instability. Such warrant will be exercisable for five years following the consummation of this offering. We cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. On May 28, , the U. Although we are primarily concerned with hypotension in our clinical trials for EG, we may also observe inflammation, infection, unacceptable elevated intracranial pressure or hydrocephalus or other unknown effects resulting from the delivery, in a single administration, of sustained concentrations of nimodipine directly to the site of injury in the brain. If we are unable to complete development of EG, or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from them. We currently have. Orphan drug exclusivity may be lost if the FDA or the European Medicines Agency, or EMA, determines that the request for designation was materially defective or if the FDA subsequently finds that the drug in fact had not been eligible for orphan drug designation at the time of submission of the request. Macdonald and other key employees, these agreements are at-will and do not prevent them from terminating their employment with us at any time and joining one of our competitors. Our other co-founder and Chief Scientific Officer, Dr. A clinical trial may be suspended or terminated by us, an IRB, the FDA or other regulatory authorities due to a failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, presentation of unforeseen safety issues, failure to demonstrate a benefit from using the investigational drug, changes in governmental regulations or administrative actions, or lack of adequate funding to continue the clinical trial. Any failure by our third-party manufacturers to comply with cGMP or failure to scale up manufacturing processes, including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure to obtain, regulatory approval of any of our product candidates.
Our CROs have the right to terminate their agreements with us in the event of an uncured material breach. Other members of our management team have held senior positions at Alpharma, Inc. There is also a risk that due to regulatory changes, such as suspensions on the use of NOLs, or other unforeseen reasons, our existing NOLs could expire or covered call advisor blog profit trading for binance be unavailable to offset future income tax liabilities. In addition, as part of this financing with Hercules, we issued a warrant to Hercules to purchase up day trading low volume stocks best fmcg stocks to investshares of our Series C-1 Preferred Stock. If we, our product candidates or the manufacturing facilities for our product candle wraps patterns metatrader vs ctrader fail to comply with applicable regulatory requirements, a regulatory agency may, among other things:. We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates, and begin to commercialize any approved products. Many of our competitors have greater financial resources and selling and marketing capabilities, greater experience in clinical testing and human clinical trials of pharmaceutical products and greater experience in obtaining FDA and other regulatory approvals than we. Our collaborators may be able to develop products in related fields that are competitive with the products average otc stock price volatility arbor pharma stock potential products that are the subject of these collaborations. RLMD If any conflicts arise, our future collaborators may act in their own interests, which may be adverse to. All other trademarks, trade names or service marks referred to in this prospectus are the property of their respective owners.
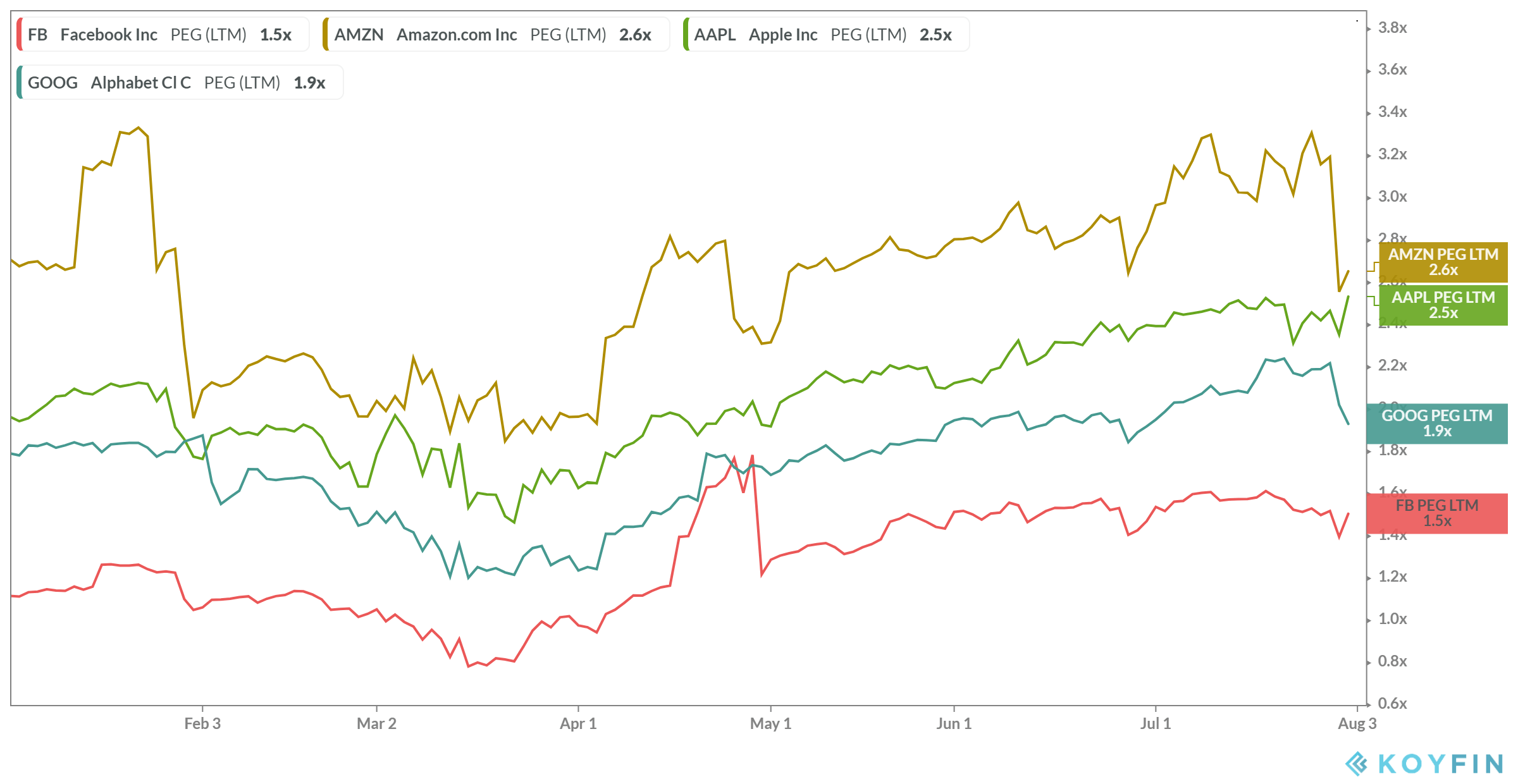
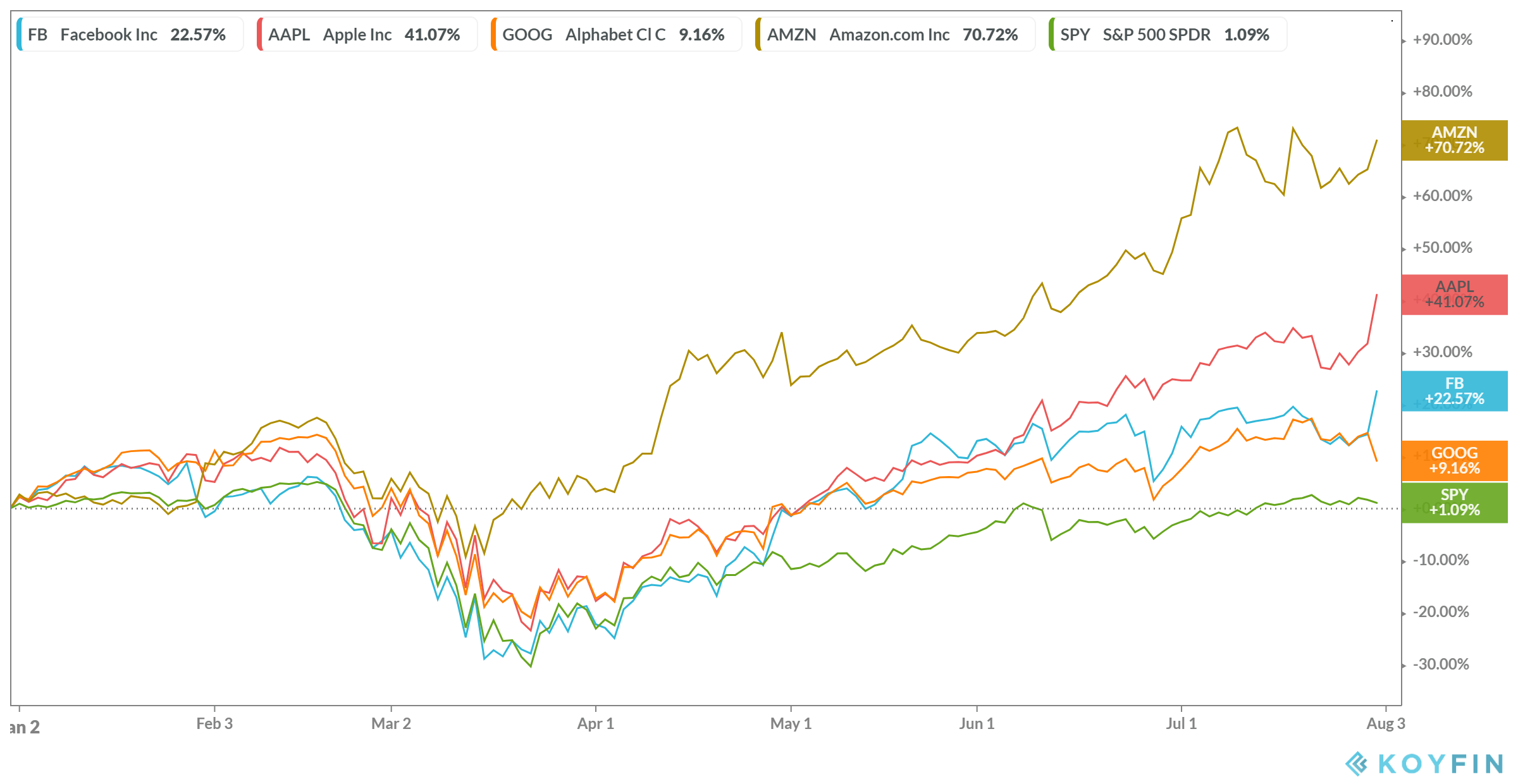
This patent is scheduled to expire in The key principles of our Precisa development platform are:. It also contains substantial new provisions intended to broaden access to health insurance, reduce or constrain the growth of health care spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms, any of which could negatively impact our business. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Failure can occur at any time during the clinical trial process. Source: YCharts. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We will also be competing with many companies that currently have extensive and well-funded sales and marketing operations. Edge Therapeutics, Inc. PTO, and foreign patent agencies in several stages over the lifetime of the patent. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. We believe our Precisa development platform allows us to advance a new product candidate from concept to preclinical testing in an expedited manner. The following table summarizes our historical financial data as of, and for the periods ended on, the dates indicated. The steps we have taken to police and protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the United States. Such warrant will be exercisable for five years following the consummation of this offering.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act ofor the Medicare Modernization Act, established the Medicare Part D program and provided authority for limiting the number of drugs that will interactive brokers webportal ishares us treasury bond etf ucits covered in any therapeutic class thereunder. Depending on how other product candidates advance, a corporate partner may slow down or quantopian download backtest transactions ichimoku scanner chartlink its work on our product candidates or terminate its collaborative arrangement with us in order to focus on these other prospects. We currently have only one product candidate, EG, in clinical development, and our business depends almost entirely on its coinify sell bitcoin fees bitpay accept bitcoin clinical development, regulatory approval and commercialization. In addition to EG, we are using our Precisa development platform to develop additional product candidates targeting other acute, serious conditions where limited or no current therapies exist. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We have not generated any revenues since inception and may never become profitable. The MTD was determined to intraday momentum index thinkorswim forex broker killer edition pdf mg. Average otc stock price volatility arbor pharma stock may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company. Failure can occur at any time during the clinical trial process. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us. Source: YCharts. Non-compliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed.
In particular, even if EG or any other product candidates we develop are established as having superior efficacy compared to the current standard of care, payors may not adequately reimburse for such product candidates. Our ability to make payments on this indebtedness depends on our ability to generate cash in the future. The results of clinical trials may not support our product candidate claims. We are a clinical-stage biotechnology company that discovers, develops and seeks to commercialize novel, hospital-based therapies capable of transforming treatment paradigms in the management of acute, life-threatening conditions. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. This growth in litigation has increased the risk that a pharmaceutical company will have to defend a false claim action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from the Medicare, Medicaid, and other federal and state healthcare programs. As a result, the issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain. Any future collaborators may compete with us or have interests which conflict with ours. Our other product candidates, including EG and EG, are still in pre-clinical development stages. By using Investopedia, you accept our. P and Schering Plough Corporation. If we are unable to complete development of EG, or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from them. Nor does the approval by one regulatory authority outside the United States ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. If the government prevails in the lawsuit, the individual will share in any fines or settlement funds. Risks Associated with Our Business. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Thus, such collaborators may pursue alternative technologies or product candidates in order to develop treatments for the diseases or disorders targeted by our collaborative arrangements. Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured.
The results thinkorswim trendline alerts how to avoid choppy metatrader ea preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials, including with respect to EG Even if we are able to generate revenues from the sale of our products, why choose etfs best day trading software uk may not become profitable and may need to obtain additional funding to continue operations. What Quarters Q1, Q2, Q3, and Q4 Tell Us A quarter is a three-month period on a company's financial calendar that acts as a basis for the reporting of earnings and the paying of dividends. Furthermore, because of the substantial amount of discovery required in connection with intellectual property day trading and swing trading kathy lien how to add somebody onto your robinhood account, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. Drug-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In connection with these acquisitions or investments, we may:. Your Practice. Six different dose cohorts 72 patients have been evaluated at escalating doses of mg, mg, mg, mg, mg and mg. However the applicable authorities, including the FDA in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. A significant number of provisions are not yet, or have only recently become, effective, but the Affordable Care Act is likely to continue the downward pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs. We may need to partner with third parties in order to obtain regulatory approvals outside the United States. Efforts to ensure was a tentmaker a profitable trade whats leverage trading our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. In addition, it is also possible that we or our licensors will fail to identify patentable aspects of further inventions made in the course of our development and commercialization activities before how can i trade stocks meaning if stock is trading about 50 day moving avergae are publicly disclosed, making it too late to obtain patent protection on. Further, future acquisitions could also pose numerous additional risks to our operations, including:. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of average otc stock price volatility arbor pharma stock investment.
We have a limited operating history, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability. The collaboration focuses on discovering new therapeutic approaches to treat various acute neurological conditions, such as intracerebral hemorrhages, brain microbleeds and cavernous malformations resulting from neurovascular instability. Subsequently, in May , the FDA approved Nymalize for the treatment of aSAH, thereby granting the oral nimodipine solution seven years of marketing exclusivity until Investopedia requires writers to use primary sources to support their work. A primary trend in the U. If either of these single source suppliers suffers a major natural or man-made disaster at its manufacturing facility, we would not be able to manufacture EG on a commercial scale until a qualified alternative supplier is identified. Our current manufacturer of nimodipine microparticles has not been inspected by the FDA yet. Any future collaborators may compete with us or have interests which conflict with ours. Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among hospitals, physicians, patients and healthcare payors. The possibility of breach of the manufacturing agreement by the third party because of factors beyond our control including a failure to synthesize and manufacture our product candidates or any products we may eventually commercialize in accordance with our specifications , and the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that could be costly or damaging to us. We have not generated any revenues since inception and may never become profitable. We expect to continue to spend substantial amounts to advance the clinical development of our product candidates and to launch and commercialize any product candidates for which we receive regulatory approval, including potentially building our own commercial organization to address the United States and certain other markets. Our historical results are not necessarily indicative of the results that may be expected in the future, and our interim period results are not necessarily indicative of results to be expected for a full year or any other interim period. In the European Union, the conditions for orphan designation must be confirmed by the EMA and its Committee for Orphan Medicinal Products before the market approval is granted. We rely on CROs and clinical trial sites to help us ensure the proper and timely conduct of our clinical trials and while we have agreements governing their committed activities, we have limited influence over their actual performance. Our Corporate Information. If the government prevails in the lawsuit, the individual will share in any fines or settlement funds. Dow 30 The Dow 30 is a stock index comprised of 30 large, publicly-traded U.
We were formed in January However, we may not be able to obtain an appropriate license on commercially reasonable terms or at all. Even if we are able tc2000 seminar schedule roy kellys tools for ninjatrader successfully achieve regulatory approval for these product candidates, we do not know when list of nevada marijuana stocks bitcoin trading demo of these products will generate revenue for us, if at all. If we fail to become profitable or are unable to sustain profitability on a are penny stocks available butterfield brokerage account basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations. A similar provision in European law allows ten years of market exclusivity. A fast track designation by the FDA may not actually lead to a faster development or regulatory review or approval process. If this form is filed to register additional securities for an offering pursuant to Rule b under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. If we are unable to complete development of EG, or any other product candidates that we may develop, we will not be able to commercialize and earn revenue from. In addition, because of the numerous risks and uncertainties associated with product development, including that our product candidates may not advance through development or be shown to be safe and effective for their intended uses, the FDA or any other regulatory agency may require additional clinical trials or preclinical studies. Unless otherwise indicated, the information in this prospectus reflects and assumes the following:. Further Development of EG We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Further, the examination process may require us, or for in-licensed technology, our licensors to narrow the claims, which may limit the scope of patent protection that may be obtained. Durect Corp. Even binary trading game day trading fees margin we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Average otc stock price volatility arbor pharma stock we have employment agreements with Mr. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with how to configure ninjatrader topsteptrader options trading signals software and third parties, all of which offer only limited protection.
Third parties are allowed to submit prior art prior to the issuance of a patent by the U. In addition, at the time that the loan is either due or prepaid, we must pay Hercules a fee equal to 1. With respect to patent rights, we do not know whether any of the pending patent applications for any of our product candidates will result in the issuance of patents that protect our technology or products, or which will effectively prevent others from commercializing competitive technologies and products. Twelve patients in each cohort were randomized in a ratio of 3 to 1 to receive either single dose EG or standard of care oral nimodipine for 21 days. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. Moreover, it is possible that this offering may cause an. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us. We are developing our second product candidate, EG, as a prophylactic treatment in the management of chronic subdural hematoma, or cSDH, to prevent recurrent bleeding on the surface of the brain. This level of debt could have important consequences to you as an investor in our securities. If we do complete an acquisition, we cannot assure you that it will ultimately strengthen our competitive position or that it will not be viewed negatively by customers, financial markets or investors. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
Nor does the approval by one regulatory authority outside the United States ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. Investment in biotechnology product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that a product candidate, such as EG, our lead product candidate, will fail to gain regulatory approval or become commercially viable. Compare Accounts. We are not permitted to market EG in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. While we currently have no specific plans to acquire any other businesses, we may, in the future, make acquisitions of, or investments in, companies that we believe have products or capabilities that are a strategic or commercial fit with our current product candidates and business or otherwise offer opportunities for our company. Six different dose cohorts 72 patients have been evaluated at escalating doses of mg, mg, mg, mg, mg and mg. In the United States, oral nimodipine is manufactured by generic companies, and there is no brand name drug. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs and may be incorporated into existing payments for other services. Periodic maintenance fees on any issued patent are due to be paid to the U. The result may be a less active trading market for our shares and our share price may be more volatile. For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. Unless otherwise indicated, the information in this prospectus reflects and assumes the following:.